Hydrocarbons important points: Hydrocarbons are the organic compounds made up of carbon and hydrogen atoms and can be classified into two different categories: aliphatic and aromatic hydrocarbons. They can be saturated or unsaturated, and the example of simplest hydrocarbon is methane.
Hydrocarbons can be used as fuels, lubricants, and in production of various chemicals such as plastics, synthetic fibers, and solvents. They are a major source of energy, but also contributes to the air pollution and climate change.
Alternative energy sources are being developed to reduce our dependence on hydrocarbons. Overall, hydrocarbons are essential building blocks for many of the products used in everyday life.
Hydrocarbons important points, Hydrocarbons important points, Hydrocarbons important points, Hydrocarbons important points, Hydrocarbons important points, Hydrocarbons important points, Hydrocarbons important points, Hydrocarbons important points
NCERT Chemistry Class 11 Chapter 13- Hydrocarbons 25 important points
There are 25 important points on Hydrocarbons –
- Hydrocarbons are the organic compounds that are composed of carbon and hydrogen atoms.
- They are the simplest type of the organic compounds and are found naturally in the fossil fuels such as petroleum, coal, and natural gas.
- Hydrocarbons can be classified into two different categories: aliphatic and aromatic hydrocarbons.
- Aliphatic hydrocarbons are composed of straight or branched chains of carbon atoms, while the aromatic hydrocarbons contain a ring of carbon atoms.
- The example of simplest hydrocarbon is methane, which is composed of one carbon atom and four hydrogen atoms.
- Hydrocarbons can be either saturated or unsaturated. Saturated hydrocarbons contains only single bonds between carbon atoms, while the unsaturated hydrocarbons contain at least one double or triple bond between carbon atoms.
- The general formula for the alkanes, which are saturated hydrocarbons, is CnH2n+2.
- Alkanes are used as the fuels and lubricants, and are also used in the production of chemicals such as plastics and synthetic fibers.
- The general formula for the alkenes, which are unsaturated hydrocarbons with one double bond, is CnH2n.
- Alkenes are used in production of plastics, synthetic rubber, and solvents.
- The general formula for the alkynes, which are unsaturated hydrocarbons with one triple bond, is CnH2n-2.
- Alkynes are used in production of plastics, synthetic rubber, and solvents.
- Aromatic hydrocarbons, also known as arenes, are composed of a ring of six carbon atoms with the alternating double bonds.
- Benzene is the example of simplest aromatic hydrocarbon and is used as a solvent and in the production of plastics, synthetic fibers, and dyes.
- Halogenated hydrocarbons are the hydrocarbons that contains one or more halogen atoms (fluorine, chlorine, bromine, or iodine).
- Halogenated hydrocarbons are used as the solvents, refrigerants, and in fire extinguishers.
- Hydrocarbons can be converted into other useful chemicals through various chemical reactions such as the cracking, reforming, and polymerization.
- Cracking is the process of breaking down the larger hydrocarbons into smaller ones by heating them in the presence of a catalyst.
- Reforming is defined as a process of rearranging the atoms in a hydrocarbon molecule to produce a different compound.
- Polymerization is a process of combining many small molecules (monomers) to form a larger molecule (polymer).
- Hydrocarbons are a major source of the energy and are used extensively as fuels for transportation, heating, and electricity generation.
- Fossil fuels, such as petroleum and coal, are composed of hydrocarbons.
- Hydrocarbons are a major contributor to the air pollution and climate change due to their combustion producing the greenhouse gases such as carbon dioxide and nitrogen oxides.
- Alternative energy sources such as wind, solar, and nuclear power are being developed to reduce our dependence on the hydrocarbons and their impact on the environment.
- Hydrocarbons are the essential building blocks for many products that are used in everyday life, including the plastics, synthetic fibers, medicines, and cosmetics
Some Important Questions From Biology Class 11
| Chapter Name | Quiz Link |
| The Living World | Play Now |
| Biological Classification | Play Now |
| Plant Kingdom | Play Now |
| Animal Kingdom | Play Now |
| Morphology of flowering plants | Play Now |
| Anatomy of flowering plants | Play Now |
| Cell: the unit of life | Play Now |
| Biomolecules | Play Now |
| Cell Cycle and cell division | Play Now |
| Transport in Plants | Play Now |
| Structural organisation in Animals | Play Now |
| Mineral nutrition | Play Now |
| Photosynthesis in higher plants | Play Now |
| Respiration in plants | Play Now |
| Plant Growth and development | Play Now |
| Digestion and Absorption | Play Now |
| Breathing and Exchange of Gases | Play Now |
| Body fluids and circulation | Play Now |
| Excretory products and their elimination | Play Now |
| Locomotion and Movement | Play Now |
| Neural Control and Coordination | Play Now |
| Chemical Coordination and Integration | Play Now |
Some Important Questions From Biology Class 12
| Chapter Name | Quiz Link |
| Reproduction in organism | Play Now |
| Sexual reproduction in flowering plant | Play Now |
| Human reproduction | Play Now |
| Reproductive health | Play Now |
| Principles of inheritance and variation | Play Now |
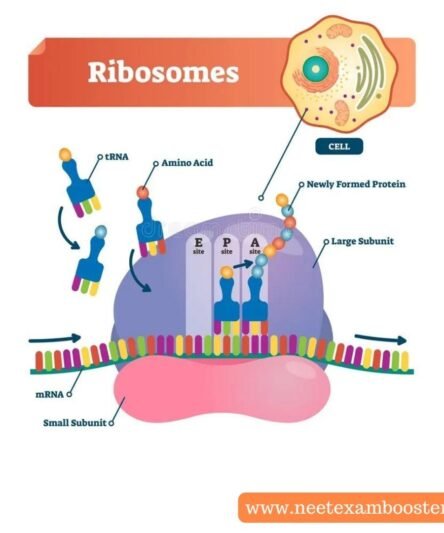
| Molecular basis of inheritance | Play Now |
| Evolution | Play Now |
| Human health and disease | Play Now |
| Strategies for enhancement in food product | Play Now |
| Microbes in human welfare | Play Now |
| Biotechnology principles and processes | Play Now |
| Biotechnology and its application | Play Now |
| Organism and population | Play Now |
| Ecosystem | Play Now |
| Biodiversity and its conservation | Play Now |
| Environment issue | Play Now |













Pingback: Chemistry Important Points For NEET For Class 11 And Class 12